गन्धक
Appearance
(Redirected from सल्फर)
| Sulfur | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
16S
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| रुप | |||||||||||||||||||||||||||||||||||||
| lemon yellow sintered microcrystals | |||||||||||||||||||||||||||||||||||||
| साधारण गुण | |||||||||||||||||||||||||||||||||||||
| नां, चिं, ल्या | sulfur, S, 16 | ||||||||||||||||||||||||||||||||||||
| उच्चारण | /ˈsʌlfər/ SUL-fər | ||||||||||||||||||||||||||||||||||||
| धातुया वर्ग | nonmetal | ||||||||||||||||||||||||||||||||||||
| ग्रुप, पिरियद, ब्लक | 16, 3, p | ||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 32.06(1) | ||||||||||||||||||||||||||||||||||||
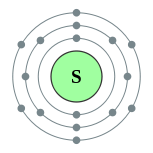
| Electron configuration | [Ne] 3s2 3p4 2, 8, 6 | ||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||
| Discovery | Chinese[१] (Before 2000BC) | ||||||||||||||||||||||||||||||||||||
| Recognized as an element by | Antoine Lavoisier (1777) | ||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | (alpha) 2.07 g·cm−3 | ||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | (beta) 1.96 g·cm−3 | ||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | (gamma) 1.92 g·cm−3 | ||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 1.819 g·cm−3 | ||||||||||||||||||||||||||||||||||||
| Melting point | 388.36 K, 115.21 °C, 239.38 °F | ||||||||||||||||||||||||||||||||||||
| Boiling point | 717.8 K, 444.6 °C, 832.3 °F | ||||||||||||||||||||||||||||||||||||
| Critical point | 1314 K, 20.7 MPa | ||||||||||||||||||||||||||||||||||||
| Heat of fusion | (mono) 1.727 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||
| Heat of vaporization | (mono) 45 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 22.75 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||
| Vapor pressure | |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||
| Oxidation states | 6, 5, 4, 3, 2, 1, -1, -2 (strongly acidic oxide) | ||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.58 (Pauling scale) | ||||||||||||||||||||||||||||||||||||
| Ionization energies (more) |
1st: 999.6 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||
| 2nd: 2252 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||
| 3rd: 3357 kJ·mol−1 | |||||||||||||||||||||||||||||||||||||
| Covalent radius | 105±3 pm | ||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 180 pm | ||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||
| Crystal structure | orthorhombic | ||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[२] | ||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (20 °C) (amorphous) 2×1015 Ω·m | ||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (amorphous) 0.205 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||
| Bulk modulus | 7.7 GPa | ||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.0 | ||||||||||||||||||||||||||||||||||||
| CAS registry number | 7704-34-9 | ||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
गन्धक छगु तत्त्व (एलेमेन्ट) ख।
लिधंसा
[सम्पादन]- ↑ Sulfur History. Georgiagulfsulfur.com. 2008-09-12 कथं।
- ↑ (2000) Magnetic susceptibility of the elements and inorganic compounds, in Handbook of Chemistry and Physics. CRC press.
स्वयादिसँ
[सम्पादन]| पिरियोडिक टेबल | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||||||
| 1 | H | He | ||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Uut | Fl | Uup | Lv | Uus | Uuo |
| परिमार्जित | ||||||||||||||||||||||||||||||||
| विकिमिडिया मंका य् थ्व विषय नाप स्वापु दुगु मिडिया दु: Sulfur |