खँलाबँला:एड्स
 | |
|---|---|
| The Red ribbon is a symbol for solidarity with HIV-positive people and those living with AIDS. | |
| ICD-10 | B24. |
| ICD-9 | 042 |
| DiseasesDB | 5938 |
| MedlinePlus | 000594 |
| eMedicine | emerg/253 |
Acquired immune deficiency syndrome or acquired immunodeficiency syndrome (AIDS or Aids) is a collection of symptoms and infections resulting from the specific damage to the immune system caused by the human immunodeficiency virus (HIV).[१] The late stage of the condition leaves individuals prone to opportunistic infections and tumors. Although treatments for AIDS and HIV exist to slow the virus's progression, there is no known cure. HIV is transmitted through direct contact of a mucous membrane or the bloodstream with a bodily fluid containing HIV, such as blood, semen, vaginal fluid, preseminal fluid, and breast milk.[२][३] This transmission can come in the form of anal, vaginal or oral sex, blood transfusion, contaminated hypodermic needles, exchange between mother and baby during pregnancy, childbirth, or breastfeeding, or other exposure to one of the above bodily fluids.
Most researchers believe that HIV originated in sub-Saharan Africa during the twentieth century;[४] it is now a pandemic, with an estimated 38.6 million people now living with the disease worldwide.[५] As of January 2006, the Joint United Nations Programme on HIV/AIDS (UNAIDS) and the World Health Organization (WHO) estimate that AIDS has killed more than 25 million people since it was first recognized on June 5, 1981, making it one of the most destructive epidemics in recorded history. In 2005 alone, AIDS claimed an estimated 2.4–3.3 million lives, of which more than 570,000 were children.[५] A third of these deaths are occurring in sub-Saharan Africa, retarding economic growth and destroying human capital. Antiretroviral treatment reduces both the mortality and the morbidity of HIV infection, but routine access to antiretroviral medication is not available in all countries.[६] HIV/AIDS stigma is more severe than that associated with other life-threatening conditions and extends beyond the disease itself to providers and even volunteers involved with the care of people living with HIV.
Infection by HIV
[सम्पादन]
AIDS is the most severe manifestation of infection with HIV. HIV is a retrovirus that primarily infects vital components of the human immune system such as CD4+ T cells (a subset of T cells), macrophages and dendritic cells. It directly and indirectly destroys CD4+ T cells. CD4+ T cells are required for the proper functioning of the immune system. When HIV kills CD4+ T cells so that there are fewer than 200 CD4+ T cells per microliter (µL) of blood, cellular immunity is lost, leading to the condition known as AIDS. Acute HIV infection progresses over time to clinical latent HIV infection and then to early symptomatic HIV infection and later to AIDS, which is identified on the basis of the amount of CD4+ T cells in the blood and the presence of certain infections.
In the absence of antiretroviral therapy, the median time of progression from HIV infection to AIDS is nine to ten years, and the median survival time after developing AIDS is only 9.2 months.[७] However, the rate of clinical disease progression varies widely between individuals, from two weeks up to 20 years. Many factors affect the rate of progression. These include factors that influence the body's ability to defend against HIV such as the infected person's general immune function.[८][९] Older people have weaker immune systems, and therefore have a greater risk of rapid disease progression than younger people. Poor access to health care and the existence of coexisting infections such as tuberculosis also may predispose people to faster disease progression.[७][१०][११] The infected person's genetic inheritance plays an important role and some people are resistant to certain strains of HIV. An example of this is people with the CCR5-Δ32 mutation are resistant to infection with certain strains of HIV.[१२] HIV is genetically variable and exists as different strains, which cause different rates of clinical disease progression.[१३][१४][१५] The use of highly active antiretroviral therapy prolongs both the median time of progression to AIDS and the median survival time.
Diagnosis
[सम्पादन]Since June 5, 1981, many definitions have been developed for epidemiological surveillance such as the Bangui definition and the 1994 expanded World Health Organization AIDS case definition. However, clinical staging of patients was not an intended use for these systems as they are neither sensitive, nor specific. In developing countries, the World Health Organization staging system for HIV infection and disease, using clinical and laboratory data, is used and in developed countries, the Centers for Disease Control (CDC) Classification System is used.
WHO disease staging system for HIV infection and disease
[सम्पादन]In 1990, the World Health Organization (WHO) grouped these infections and conditions together by introducing a staging system for patients infected with HIV-1.[१६] An update took place in September 2005. Most of these conditions are opportunistic infections that are easily treatable in healthy people.
- Stage I: HIV disease is asymptomatic and not categorized as AIDS
- Stage II: includes minor mucocutaneous manifestations and recurrent upper respiratory tract infections
- Stage III: includes unexplained chronic diarrhea for longer than a month, severe bacterial infections and pulmonary tuberculosis
- Stage IV: includes toxoplasmosis of the brain, candidiasis of the esophagus, trachea, bronchi or lungs and Kaposi's sarcoma; these diseases are indicators of AIDS.
CDC classification system for HIV infection
[सम्पादन]In the beginning, the Centers for Disease Control and Prevention (CDC) did not have an official name for the disease, often referring to it by way of the diseases that were associated with it, for example, lymphadenopathy, the disease after which the discoverers of HIV originally named the virus.[१७][१८] They also used Kaposi's Sarcoma and Opportunistic Infections, the name by which a task force had been set up in 1981.[१९] In the general press, the term GRID, which stood for Gay Related Immune Disease, had been coined.[२०] However, after determining that AIDS was not isolated to the homosexual community,[१९] the term GRID became redundant and AIDS was introduced at a meeting in July 1982.[२१] By September 1982 the CDC started using the name AIDS, and properly defined the illness.[२२] In 1993, the CDC expanded their definition of AIDS to include all HIV positive people with a CD4+ T cell count below 200 per µL of blood or 14% of all lymphocytes.[२३] The majority of new AIDS cases in developed countries use either this definition or the pre-1993 CDC definition. The AIDS diagnosis still stands even if, after treatment, the CD4+ T cell count rises to above 200 per µL of blood or other AIDS-defining illnesses are cured.
HIV test
[सम्पादन]Many people are unaware that they are infected with HIV.[२४] Less than 1% of the sexually active urban population in Africa has been tested, and this proportion is even lower in rural populations. Furthermore, only 0.5% of pregnant women attending urban health facilities are counseled, tested or receive their test results. Again, this proportion is even lower in rural health facilities.[२४] Therefore, donor blood and blood products used in medicine and medical research are screened for HIV. Typical HIV tests, including the HIV enzyme immunoassay and the Western blot assay, detect HIV antibodies in serum, plasma, oral fluid, dried blood spot or urine of patients. However, the window period (the time between initial infection and the development of detectable antibodies against the infection) can vary. This is why it can take 3–6 months to seroconvert and test positive. Commercially available tests to detect other HIV antigens, HIV-RNA, and HIV-DNA in order to detect HIV infection prior to the development of detectable antibodies are available. For the diagnosis of HIV infection these assays are not specifically approved, but are nonetheless routinely used in developed countries.
Symptoms and complications
[सम्पादन]
The symptoms of AIDS are primarily the result of conditions that do not normally develop in individuals with healthy immune systems. Most of these conditions are infections caused by bacteria, viruses, fungi and parasites that are normally controlled by the elements of the immune system that HIV damages. Opportunistic infections are common in people with AIDS.[२५] HIV affects nearly every organ system. People with AIDS also have an increased risk of developing various cancers such as Kaposi's sarcoma, cervical cancer and cancers of the immune system known as lymphomas.
Additionally, people with AIDS often have systemic symptoms of infection like fevers, sweats (particularly at night), swollen glands, chills, weakness, and weight loss.[२६][२७] After the diagnosis of AIDS is made, the current average survival time with antiretroviral therapy (as of 2005) is estimated to be more than 5 years,[२८] but because new treatments continue to be developed and because HIV continues to evolve resistance to treatments, estimates of survival time are likely to continue to change. Without antiretroviral therapy, death normally occurs within a year.[७] Most patients die from opportunistic infections or malignancies associated with the progressive failure of the immune system.[२९]
The rate of clinical disease progression varies widely between individuals and has been shown to be affected by many factors such as host susceptibility and immune function[८][९][१२] health care and co-infections,[७][२९] as well as factors relating to the viral strain.[१४][३०][३१] The specific opportunistic infections that AIDS patients develop depend in part on the prevalence of these infections in the geographic area in which the patient lives.
Major pulmonary illnesses
[सम्पादन]
- Pneumocystis jiroveci pneumonia (originally known as Pneumocystis carinii pneumonia, often-abbreviated PCP) is relatively rare in healthy, immunocompetent people, but common among HIV-infected individuals. Before the advent of effective diagnosis, treatment and routine prophylaxis in Western countries, it was a common immediate cause of death. In developing countries, it is still one of the first indications of AIDS in untested individuals, although it does not generally occur unless the CD4 count is less than 200 per µL.[३२]
- Tuberculosis (TB) is unique among infections associated with HIV because it is transmissible to immunocompetent people via the respiratory route, is easily treatable once identified, may occur in early-stage HIV disease, and is preventable with drug therapy. However, multidrug resistance is a potentially serious problem. Even though its incidence has declined because of the use of directly observed therapy and other improved practices in Western countries, this is not the case in developing countries where HIV is most prevalent. In early-stage HIV infection (CD4 count >300 cells per µL), TB typically presents as a pulmonary disease. In advanced HIV infection, TB often presents atypically with extrapulmonary (systemic) disease a common feature. Symptoms are usually constitutional and are not localized to one particular site, often affecting bone marrow, bone, urinary and gastrointestinal tracts, liver, regional lymph nodes, and the central nervous system.[३३] Alternatively, symptoms may relate more to the site of extrapulmonary involvement.
Major gastro-intestinal illnesses
[सम्पादन]- Esophagitis is an inflammation of the lining of the lower end of the esophagus (gullet or swallowing tube leading to the stomach). In HIV infected individuals, this is normally due to fungal (candidiasis) or viral (herpes simplex-1 or cytomegalovirus) infections. In rare cases, it could be due to mycobacteria.[३४]
- Unexplained chronic diarrhea in HIV infection is due to many possible causes, including common bacterial (Salmonella, Shigella, Listeria, Campylobacter, or Escherichia coli) and parasitic infections; and uncommon opportunistic infections such as cryptosporidiosis, microsporidiosis, Mycobacterium avium complex (MAC) and cytomegalovirus (CMV) colitis. In some cases, diarrhea may be a side effect of several drugs used to treat HIV, or it may simply accompany HIV infection, particularly during primary HIV infection. It may also be a side effect of antibiotics used to treat bacterial causes of diarrhea (common for Clostridium difficile). In the later stages of HIV infection, diarrhea is thought to be a reflection of changes in the way the intestinal tract absorbs nutrients, and may be an important component of HIV-related wasting.[३५]
Major neurological illnesses
[सम्पादन]- Toxoplasmosis is a disease caused by the single-celled parasite called Toxoplasma gondii; it usually infects the brain causing toxoplasma encephalitis but it can infect and cause disease in the eyes and lungs.[३६]
- Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease, in which the gradual destruction of the myelin sheath covering the axons of nerve cells impairs the transmission of nerve impulses. It is caused by a virus called JC virus which occurs in 70% of the population in latent form, causing disease only when the immune system has been severely weakened, as is the case for AIDS patients. It progresses rapidly, usually causing death within months of diagnosis.[३७]
- AIDS dementia complex (ADC) is a metabolic encephalopathy induced by HIV infection and fueled by immune activation of HIV infected brain macrophages and microglia which secrete neurotoxins of both host and viral origin.[३८] Specific neurological impairments are manifested by cognitive, behavioral, and motor abnormalities that occur after years of HIV infection and is associated with low CD4+ T cell levels and high plasma viral loads. Prevalence is 10–20% in Western countries[३९] but only 1–2% of HIV infections in India.[४०][४१] This difference is possibly due to the HIV subtype in India.
- Cryptococcal meningitis is an infection of the meninx (the membrane covering the brain and spinal cord) by the fungus Cryptococcus neoformans. It can cause fevers, headache, fatigue, nausea, and vomiting. Patients may also develop seizures and confusion; left untreated, it can be lethal.
Major HIV-associated malignancies
[सम्पादन]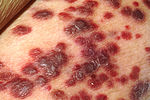
Patients with HIV infection have substantially increased incidence of several malignant cancers. This is primarily due to co-infection with an oncogenic DNA virus, especially Epstein-Barr virus (EBV), Kaposi's sarcoma-associated herpesvirus (KSHV), and human papillomavirus (HPV).[४२][४३] The following confer a diagnosis of AIDS when they occur in an HIV-infected person.
- Kaposi's sarcoma (KS) is the most common tumor in HIV-infected patients. The appearance of this tumor in young homosexual men in 1981 was one of the first signals of the AIDS epidemic. Caused by a gammaherpes virus called Kaposi's sarcoma-associated herpes virus (KSHV), it often appears as purplish nodules on the skin, but can affect other organs, especially the mouth, gastrointestinal tract, and lungs.
- High-grade B cell lymphomas such as Burkitt's lymphoma, Burkitt's-like lymphoma, diffuse large B-cell lymphoma (DLBCL), and primary central nervous system lymphoma present more often in HIV-infected patients. These particular cancers often foreshadow a poor prognosis. In some cases these lymphomas are AIDS-defining. Epstein-Barr virus (EBV) or KSHV cause many of these lymphomas.
- Cervical cancer in HIV-infected women is considered AIDS-defining. It is caused by human papillomavirus (HPV).
In addition to the AIDS-defining tumors listed above, HIV-infected patients are at increased risk of certain other tumors, such as Hodgkin's disease and anal and rectal carcinomas. However, the incidence of many common tumors, such as breast cancer or colon cancer, does not increase in HIV-infected patients. In areas where HAART is extensively used to treat AIDS, the incidence of many AIDS-related malignancies has decreased, but at the same time malignant cancers overall have become the most common cause of death of HIV-infected patients.[४४]
Other opportunistic infections
[सम्पादन]AIDS patients often develop opportunistic infections that present with non-specific symptoms, especially low-grade fevers and weight loss. These include infection with Mycobacterium avium-intracellulare and cytomegalovirus (CMV). CMV can cause colitis, as described above, and CMV retinitis can cause blindness. Penicilliosis due to Penicillium marneffei is now the third most common opportunistic infection (after extrapulmonary tuberculosis and cryptococcosis) in HIV-positive individuals within the endemic area of Southeast Asia.[४५]
Transmission and prevention
[सम्पादन]| Exposure Route | Estimated infections per 10,000 exposures to an infected source | |||
|---|---|---|---|---|
| Blood Transfusion | 9,000[४७] | |||
| Childbirth | 2,500[४८] | |||
| Needle-sharing injection drug use | 67[४९] | |||
| Receptive anal intercourse* | 50[५०][५१] | |||
| Percutaneous needle stick | 30[५२] | |||
| Receptive penile-vaginal intercourse* | 10[५०][५१][५३] | |||
| Insertive anal intercourse* | 6.5[५०][५१] | |||
| Insertive penile-vaginal intercourse* | 5[५०][५१] | |||
| Receptive oral intercourse* | 1[५१]§ | |||
| Insertive oral intercourse* | 0.5[५१]§ | |||
| * assuming no condom use § Source refers to oral intercourse performed on a man | ||||
The three main transmission routes of HIV are sexual contact, exposure to infected body fluids or tissues, and from mother to fetus or child during perinatal period. It is possible to find HIV in the saliva, tears, and urine of infected individuals, but there are no recorded cases of infection by these secretions, and the risk of infection is negligible.
Sexual contact
[सम्पादन]The majority of HIV infections are acquired through unprotected sexual relations between partners, one of whom has HIV. Sexual transmission occurs with the contact between sexual secretions of one partner with the rectal, genital or oral mucous membranes of another. Unprotected receptive sexual acts are riskier than unprotected insertive sexual acts, with the risk for transmitting HIV from an infected partner to an uninfected partner through unprotected insertive anal intercourse greater than the risk for transmission through vaginal intercourse or oral sex. Oral sex is not without its risks as HIV is transmissible through both insertive and receptive oral sex.[५४] The risk of HIV transmission from exposure to saliva is considerably smaller than the risk from exposure to semen; contrary to popular belief, one would have to swallow gallons of saliva from a carrier to run a significant risk of becoming infected.[५५]
Approximately 30% of women in ten countries representing "diverse cultural, geographical and urban/rural settings" report that their first sexual experience was forced or coerced, making sexual violence a key driver of the HIV/AIDS pandemic.[५६] Sexual assault greatly increases the risk of HIV transmission as protection is rarely employed and physical trauma to the vaginal cavity frequently occurs which facilitates the transmission of HIV.[५७]
Sexually transmitted infections (STI) increase the risk of HIV transmission and infection because they cause the disruption of the normal epithelial barrier by genital ulceration and/or microulceration; and by accumulation of pools of HIV-susceptible or HIV-infected cells (lymphocytes and macrophages) in semen and vaginal secretions. Epidemiological studies from sub-Saharan Africa, Europe and North America have suggested that there is approximately a four times greater risk of becoming infected with HIV in the presence of a genital ulcer such as those caused by syphilis and/or chancroid. There is also a significant though lesser increased risk in the presence of STIs such as gonorrhea, Chlamydial infection and trichomoniasis which cause local accumulations of lymphocytes and macrophages.[५८]
Transmission of HIV depends on the infectiousness of the index case and the susceptibility of the uninfected partner. Infectivity seems to vary during the course of illness and is not constant between individuals. An undetectable plasma viral load does not necessarily indicate a low viral load in the seminal liquid or genital secretions. Each 10-fold increment of blood plasma HIV RNA is associated with an 81% increased rate of HIV transmission.[५८][५९] Women are more susceptible to HIV-1 infection due to hormonal changes, vaginal microbial ecology and physiology, and a higher prevalence of sexually transmitted diseases.[६०][६१] People who are infected with HIV can still be infected by other, more virulent strains.
During a sexual act, only male or female condoms can reduce the chances of infection with HIV and other STDs and the chances of becoming pregnant. The best evidence to date indicates that typical condom use reduces the risk of heterosexual HIV transmission by approximately 80% over the long-term, though the benefit is likely to be higher if condoms are used correctly on every occasion.[६२] The effective use of condoms and screening of blood transfusion in North America, Western and Central Europe is credited with contributing to the low rates of AIDS in these regions. Promoting condom use, however, has often proved controversial and difficult. Many religious groups, most noticeably the Catholic Church, have opposed the use of condoms on religious grounds, and have sometimes seen condom promotion as an affront to the promotion of marriage, monogamy and sexual morality. Defenders of the Catholic Church's role in AIDS and general STD prevention state that, while they may be against the use of contraception, they are strong advocates of abstinence outside marriage.[६३] This attitude is also found among some health care providers and policy makers in sub-Saharan African nations, where HIV and AIDS prevalence is extremely high.[६४] They also believe that the distribution and promotion of condoms is tantamount to promoting sex amongst the youth and sending the wrong message to uninfected individuals. However, no evidence has been produced that promotion of condom use increases sexual promiscuity,[६५] and abstinence-only programs have been unsuccessful both in changing sexual behavior and in reducing HIV transmission.[६६] Evaluations of several abstinence-only programs in the US showed a negative impact on the willingness of youths to use contraceptives, due to the emphasis on contraceptives' failure rates.[६७]
The male latex condom, if used correctly without oil-based lubricants, is the single most effective available technology to reduce the sexual transmission of HIV and other sexually transmitted infections. Manufacturers recommend that oil-based lubricants such as petroleum jelly, butter, and lard not be used with latex condoms, because they dissolve the latex, making the condoms porous. If necessary, manufacturers recommend using water-based lubricants. Oil-based lubricants can however be used with polyurethane condoms.[६८] Latex condoms degrade over time, making them porous, which is why condoms have expiration dates. In Europe and the United States, condoms have to conform to European (EC 600) or American (D3492) standards to be considered protective against HIV transmission.
The female condom is an alternative to the male condom and is made from polyurethane, which allows it to be used in the presence of oil-based lubricants. They are larger than male condoms and have a stiffened ring-shaped opening, and are designed to be inserted into the vagina. The female condom contains an inner ring, which keeps the condom in place inside the vagina — inserting the female condom requires squeezing this ring. However, at present availability of female condoms is very low and the price remains prohibitive for many women. Preliminary studies suggest that, where female condoms are available, overall protected sexual acts increase relative to unprotected sexual acts, making them an important HIV prevention strategy that must be scaled-up.[६९]
With consistent and correct use of condoms, there is a very low risk of HIV infection. Studies on couples where one partner is infected show that with consistent condom use, HIV infection rates for the uninfected partner are below 1% per year.[७०]
The United States government and health organizations both endorse the ABC Approach to lower the risk of acquiring AIDS during sex:
- Abstinence or delay of sexual activity, especially for youth,
- Being faithful, especially for those in committed relationships,
- Condom use, for those who engage in risky behavior.
This approach has been very successful in Uganda, where HIV prevalence has decreased from 15% to 5%. However, more has been done than just this. As Edward Green, a Harvard medical anthropologist, put it, "Uganda has pioneered approaches towards reducing stigma, bringing discussion of sexual behavior out into the open, involving HIV-infected people in public education, persuading individuals and couples to be tested and counseled, improving the status of women, involving religious organizations, enlisting traditional healers, and much more." However, criticism of the ABC approach is widespread because a faithful partner of an unfaithful partner is at risk of contracting HIV and that discrimination against women and girls is so great that they are without voice in almost every area of their lives.[७१] Other programs and initiatives promote condom use more heavily. Condom use is an integral part of the CNN Approach. This is:
- Condom use, for those who engage in risky behavior,
- Needles, use clean ones,
- Negotiating skills; negotiating safer sex with a partner and empowering women to make smart choices.
In December 2006, the last of three large, randomized trials confirmed that male circumcision lowers the risk of HIV infection among heterosexual African men by around 50%. It is expected that this intervention will be actively promoted in many of the countries worst affected by HIV, although doing so will involve confronting a number of practical, cultural and attitudinal issues. Some experts fear that a lower perception of vulnerability among circumcised men may result in more sexual risk-taking behavior, thus negating its preventive effects.[७२] Furthermore, South African medical experts are concerned that the repeated use of unsterilized blades in the ritual circumcision of adolescent boys may be spreading HIV.[७३]
Exposure to infected body fluids
[सम्पादन]This transmission route is particularly relevant to intravenous drug users, hemophiliacs and recipients of blood transfusions and blood products. Sharing and reusing syringes contaminated with HIV-infected blood represents a major risk for infection with not only HIV, but also hepatitis B and hepatitis C. Needle sharing is the cause of one third of all new HIV-infections and 50% of hepatitis C infections in North America, China, and Eastern Europe. The risk of being infected with HIV from a single prick with a needle that has been used on an HIV-infected person is thought to be about 1 in 150 (see table above). Post-exposure prophylaxis with anti-HIV drugs can further reduce that small risk.[७४] Health care workers (nurses, laboratory workers, doctors etc) are also concerned, although more rarely. This route can affect people who give and receive tattoos and piercings. Universal precautions are frequently not followed in both sub-Saharan Africa and much of Asia because of both a shortage of supplies and inadequate training. The WHO estimates that approximately 2.5% of all HIV infections in sub-Saharan Africa are transmitted through unsafe healthcare injections.[७५] Because of this, the United Nations General Assembly, supported by universal medical opinion on the matter, has urged the nations of the world to implement universal precautions to prevent HIV transmission in health care settings.[७६][७७]
The risk of transmitting HIV to blood transfusion recipients is extremely low in developed countries where improved donor selection and HIV screening is performed. However, according to the WHO, the overwhelming majority of the world's population does not have access to safe blood and "between 5% and 10% of HIV infections worldwide are transmitted through the transfusion of infected blood and blood products".[७८]
Medical workers who follow universal precautions or body-substance isolation, such as wearing latex gloves when giving injections and washing the hands frequently, can help prevent infection by HIV.
All AIDS-prevention organizations advise drug-users not to share needles and other material required to prepare and take drugs (including syringes, cotton balls, the spoons, water for diluting the drug, straws, crack pipes, etc). It is important that people use new or properly sterilized needles for each injection. Information on cleaning needles using bleach is available from health care and addiction professionals and from needle exchanges. In some developed countries, clean needles are available free in some cities, at needle exchanges or safe injection sites. Additionally, many nations have decriminalized needle possession and made it possible to buy injection equipment from pharmacists without a prescription.
Mother-to-child transmission (MTCT)
[सम्पादन]The transmission of the virus from the mother to the child can occur in utero during the last weeks of pregnancy and at childbirth. In the absence of treatment, the transmission rate between the mother to the child during pregnancy, labor and delivery is 25%. However, when the mother has access to antiretroviral therapy and gives birth by caesarean section, the rate of transmission is just 1%.[४८] A number of factors influence the risk of infection, particularly the viral load of the mother at birth (the higher the load, the higher the risk). Breastfeeding increases the risk of transmission by 10–15%. This risk depends on clinical factors and may vary according to the pattern and duration of breast-feeding.
Studies have shown that antiretroviral drugs, caesarean delivery and formula feeding reduce the chance of transmission of HIV from mother to child.[७९] Current recommendations state that when replacement feeding is acceptable, feasible, affordable, sustainable and safe, HIV-infected mothers should avoid breast-feeding their infant. However, if this is not the case, exclusive breast-feeding is recommended during the first months of life and discontinued as soon as possible.[५] In 2005, around 700,000 children under 15 contracted HIV, mainly through MTCT, with 630,000 of these infections occurring in Africa.[८०] Of the estimated 2.3 million [1.7–3.5 million] children currently living with HIV, 2 million (almost 90%) live in sub-Saharan Africa.[५]
Prevention strategies are well known in developed countries, however, recent epidemiological and behavioral studies in Europe and North America have suggested that a substantial minority of young people continue to engage in high-risk practices and that despite HIV/AIDS knowledge, young people underestimate their own risk of becoming infected with HIV.[८१] However, transmission of HIV between intravenous drug users has clearly decreased, and HIV transmission by blood transfusion has become quite rare in developed countries.
Treatment
[सम्पादन]- See also HIV Treatment.
There is currently no vaccine or cure for HIV or AIDS. The only known methods of prevention are based on avoiding exposure to the virus or, failing that, an antiretroviral treatment directly after a highly significant exposure, called post-exposure prophylaxis (PEP).[७४] PEP has a very demanding four week schedule of dosage. It also has very unpleasant side effects including diarrhea, malaise, nausea and fatigue.[८२]



Current treatment for HIV infection consists of highly active antiretroviral therapy, or HAART.[८३] This has been highly beneficial to many HIV-infected individuals since its introduction in 1996 when the protease inhibitor-based HAART initially became available.[६] Current optimal HAART options consist of combinations (or "cocktails") consisting of at least three drugs belonging to at least two types, or "classes," of anti-retroviral agents. Typical regimens consist of two nucleoside analogue reverse transcriptase inhibitors (NARTIs or NRTIs) plus either a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor (NNRTI). Because HIV disease progression in children is more rapid than in adults, and laboratory parameters are less predictive of risk for disease progression, particularly for young infants, treatment recommendations are more aggressive for children than for adults.[८४] In developed countries where HAART is available, doctors assess the viral load, rapidity in CD4 decline, and patient readiness while deciding when to recommend initiating treatment.[८५]
HAART allows the stabilization of the patient’s symptoms and viremia, but it neither cures the patient of HIV, nor alleviates the symptoms, and high levels of HIV-1, often HAART resistant, return once treatment is stopped.[८६][८७] Moreover, it would take more than the lifetime of an individual to be cleared of HIV infection using HAART.[८८] Despite this, many HIV-infected individuals have experienced remarkable improvements in their general health and quality of life, which has led to the plummeting of HIV-associated morbidity and mortality.[८९][९०][९१] In the absence of HAART, progression from HIV infection to AIDS occurs at a median of between nine to ten years and the median survival time after developing AIDS is only 9.2 months.[७] HAART is thought to increase survival time by between 4 and 12 years.[९२][९३] This average reflects the fact that for some patients — and in many clinical cohorts this may be more than fifty percent of patients — HAART achieves far less than optimal results. This is due to a variety of reasons such as medication intolerance/side effects, prior ineffective antiretroviral therapy and infection with a drug-resistant strain of HIV. However, non-adherence and non-persistence with antiretroviral therapy is the major reason most individuals fail to get any benefit from and develop resistance to HAART.[९४] The reasons for non-adherence and non-persistence with HAART are varied and overlapping. Major psychosocial issues, such as poor access to medical care, inadequate social supports, psychiatric disease and drug abuse contribute to non-adherence. The complexity of these HAART regimens, whether due to pill number, dosing frequency, meal restrictions or other issues along with side effects that create intentional non-adherence also has a weighty impact.[९५][९६][९७] The side effects include lipodystrophy, dyslipidaemia, insulin resistance, an increase in cardiovascular risks and birth defects.[९८][९९]
Anti-retroviral drugs are expensive, and the majority of the world's infected individuals do not have access to medications and treatments for HIV and AIDS.[१००] Research to improve current treatments includes decreasing side effects of current drugs, further simplifying drug regimens to improve adherence, and determining the best sequence of regimens to manage drug resistance. However, only a vaccine is postulated to be able to halt the pandemic. This is because a vaccine would possibly cost less, thus being affordable for developing countries, and would not require daily treatments.[१००] However, after over 20 years of research, HIV-1 remains a difficult target for a vaccine.[१००]
A number of studies have shown that measures to prevent opportunistic infections can be beneficial when treating patients with HIV infection or AIDS. Vaccination against hepatitis A and B is advised for patients who are not infected with these viruses and are at risk of becoming infected.[१०१] Patients with substantial immunosuppression are also advised to receive prophylactic therapy for Pneumocystis jiroveci pneumonia (PCP), and many patients may benefit from prophylactic therapy for toxoplasmosis and Cryptococcus meningitis as well.[८२] Daily multivitamin and mineral supplements have been found to reduce HIV disease progression among men and women. This could become an important low-cost intervention provided during early HIV disease to prolong the time before antiretroviral therapy is required.[१०२][१०३]
Various forms of alternative medicine have been used to treat symptoms or alter the course of the disease.[१०४] In the first decade of the epidemic when no useful conventional treatment was available, a large number of people with AIDS experimented with alternative therapies. The definition of "alternative therapies" in AIDS has changed since that time. Then, the phrase often referred to community-driven treatments, untested by government or pharmaceutical company research, that some hoped would directly suppress the virus or stimulate immunity against it. Examples of alternative medicine that people hoped would improve their symptoms or their quality of life include massage, stress management, herbal and flower remedies and acupuncture;[१०४] when used with conventional treatment, many now refer to these as "complementary" approaches. Despite the widespread use of complementary and alternative medicine by people living with HIV/AIDS, the effectiveness of these therapies has not been established.[१०५]
Epidemiology
[सम्पादन]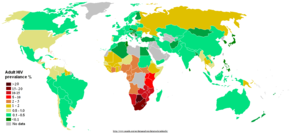
UNAIDS and the WHO estimate that AIDS has killed more than 25 million people since it was first recognized in 1981, making it one of the most destructive epidemics in recorded history. Despite recent, improved access to antiretroviral treatment and care in many regions of the world, the AIDS epidemic claimed an estimated 2.8 million (between 2.4 and 3.3 million) lives in 2005 of which more than half a million (570,000) were children.[५]
Globally, between 33.4 and 46 million people currently live with HIV.[५] In 2005, between 3.4 and 6.2 million people were newly infected and between 2.4 and 3.3 million people with AIDS died, an increase from 2003 and the highest number since 1981.[५]
Sub-Saharan Africa remains by far the worst affected region, with an estimated 21.6 to 27.4 million people currently living with HIV. Two million [1.5–3.0 million] of them are children younger than 15 years of age. More than 64% of all people living with HIV are in sub-Saharan Africa, as are more than three quarters (76%) of all women living with HIV. In 2005, there were 12.0 million [10.6–13.6 million] AIDS orphans living in sub-Saharan Africa 2005.[५] South & South East Asia are second worst affected with 15%. AIDS accounts for the deaths of 500,000 children in this region. Two-thirds of HIV/AIDS infections in Asia occur in India, with an estimated 5.7 million infections (estimated 3.4 – 9.4 million) (0.9% of population), surpassing South Africa's estimated 5.5 million (4.9–6.1 million) (11.9% of population) infections, making it the country with the highest number of HIV infections in the world.[१०६] In the 35 African nations with the highest prevalence, average life expectancy is 48.3 years— 6.5 years less than it would be without the disease.[१०७]
The latest evaluation report of the World Bank's Operations Evaluation Department assesses the effectiveness of the World Bank's country-level HIV/AIDS assistance, defined as policy dialogue, analytic work, and lending, with the explicit objective of reducing the scope or impact of the AIDS epidemic.[१०८] This is the first comprehensive evaluation of the World Bank's HIV/AIDS support to countries, from the beginning of the epidemic through mid-2004. Because the Bank's assistance is for implementation of government programs by government, it provides important insights on how national AIDS programs can be made more effective.
The development of HAART as effective therapy for HIV infection and AIDS has substantially reduced the death rate from this disease in those areas where it is widely available. This has created the misperception that the disease has gone away. In fact, as the life expectancy of persons with AIDS has increased in countries where HAART is widely used, the number of persons living with AIDS has increased substantially. In the United States, the number of persons with AIDS increased from about 35,000 in 1988 to over 220,000 in 1996.[१०९]
In Africa, the number of MTCT and the prevalence of AIDS is beginning to reverse decades of steady progress in child survival. Countries such as Uganda are attempting to curb the MTCT epidemic by offering VCT (voluntary counseling and testing), PMTCT (prevention of mother-to-child transmission) and ANC (ante-natal care) services, which include the distribution of antiretroviral therapy.
Economic impact
[सम्पादन]HIV and AIDS retard economic growth by destroying human capital. UNAIDS has predicted outcomes for sub-Saharan Africa to the year 2025. These range from a plateau and eventual decline in deaths beginning around 2012 to a catastrophic continual growth in the death rate with potentially 90 million cases of infection.[५]
Without proper nutrition, health care and medicine that is available in developed countries, large numbers of people in these countries are falling victim to AIDS. They will not only be unable to work, but will also require significant medical care. The forecast is that this will likely cause a collapse of economies and societies in the region. In some heavily infected areas, the epidemic has left behind many orphans cared for by elderly grandparents.
The increased mortality in this region will result in a smaller skilled population and labor force.[११०] This smaller labor force will be predominantly young people, with reduced knowledge and work experience leading to reduced productivity. An increase in workers’ time off to look after sick family members or for sick leave will also lower productivity. Increased mortality will also weaken the mechanisms that generate human capital and investment in people, through loss of income and the death of parents.[११०] By killing off mainly young adults, AIDS seriously weakens the taxable population, reducing the resources available for public expenditures such as education and health services not related to AIDS resulting in increasing pressure for the state's finances and slower growth of the economy. This results in a slower growth of the tax base, an effect that will be reinforced if there are growing expenditures on treating the sick, training (to replace sick workers), sick pay and caring for AIDS orphans. This is especially true if the sharp increase in adult mortality shifts the responsibility and blame from the family to the government in caring for these orphans.
On the level of the household, AIDS results in both the loss of income and increased spending on healthcare by the household. The income effects of this lead to spending reduction as well as a substitution effect away from education and towards healthcare and funeral spending. A study in Côte d'Ivoire showed that households with an HIV/AIDS patient spent twice as much on medical expenses as other households.[१११]
UNAIDS, WHO and the United Nations Development Programme have documented a correlation between the decreasing life expectancies and the lowering of gross national product in many African countries with prevalence rates of 10% or more. Indeed, since 1992 predictions that AIDS would slow economic growth in these countries have been published. The degree of impact depended on assumptions about the extent to which illness would be funded by savings and who would be infected.[११२] Conclusions reached from models of the growth trajectories of 30 sub-Saharan economies over the period 1990–2025 were that the economic growth rates of these countries would be between 0.56 and 1.47% lower. The impact on gross domestic product (GDP) per capita was less conclusive. However, in 2000, the rate of growth of Africa's per capita GDP was in fact reduced by 0.7% per year from 1990–1997 with a further 0.3% per year lower in countries also affected by malaria.[११३] The forecast now is that the growth of GDP for these countries will undergo a further reduction of between 0.5 and 2.6% per annum.[११०] However, these estimates may be an underestimate, as they do not look at the effects on output per capita.[११४]
Many governments in sub-Saharan Africa denied that there was a problem for years, and are only now starting to work towards solutions. Underfunding is a problem in all areas of HIV prevention when compared to even conservative estimates of the problems.
The launching of the world's first official HIV/AIDS Toolkit in Zimbabwe on October 3 2006 is a product of collaborative work between the International Federation of Red Cross and Red Crescent Societies, World Health Organization and the Southern Africa HIV/AIDS Information Dissemination Service. It is for the strengthening of people living with HIV/AIDS and nurses by minimal external support. The package, which is in form of eight modules focusing on basic facts about HIV and AIDS, was pre-tested in Zimbabwe in March 2006 to determine its adaptability. It disposes, among other things, categorized guidelines on clinical management, education and counseling of AIDS victims at community level.[११५]
The Copenhagen Consensus is a project that seeks to establish priorities for advancing global welfare using methodologies based on the theory of welfare economics. The participants are all economists, with the focus of the project being a rational prioritization based on economic analysis. The project is based on the contention that, in spite of the billions of dollars spent on global challenges by the United Nations, the governments of wealthy nations, foundations, charities, and non-governmental organizations, the money spent on problems such as malnutrition and climate change is not sufficient to meet many internationally-agreed targets. The highest priority was assigned to implementing new measures to prevent the spread of HIV and AIDS. The economists estimated that an investment of $27 billion could avert nearly 30 million new infections by 2010.
Stigma
[सम्पादन]AIDS stigma exists around the world in a variety of ways, including ostracism, rejection, discrimination and avoidance of HIV infected people; compulsory HIV testing without prior consent or protection of confidentiality; violence against HIV infected individuals or people who are perceived to be infected with HIV; and the quarantine of HIV infected individuals.[११६] Stigma-related violence or the fear of violence prevents many people from seeking HIV testing, returning for their results, or securing treatment, possibly turning what could be a manageable chronic illness into a death sentence and perpetuating the spread of HIV.[११७]
AIDS stigma has been further divided into the following three categories:
- Instrumental AIDS stigma—a reflection of the fear and apprehension that are likely to be associated with any deadly and transmissible illness.[११८]
- Symbolic AIDS stigma—the use of HIV/AIDS to express attitudes toward the social groups or lifestyles perceived to be associated with the disease.[११८]
- Courtesy AIDS stigma—stigmatization of people connected to the issue of HIV/AIDS or HIV- positive people.[११९]
Often, AIDS stigma is expressed in conjunction with one or more other stigmas, particularly those associated with homosexuality, bisexuality, promiscuity, and intravenous drug use.
In many developed countries, there is an association between AIDS and homosexuality or bisexuality, and this association is correlated with higher levels of sexual prejudice such as anti-homosexual attitudes.[१२०] There is also a perceived association between all male-male sexual behavior and AIDS, even sex between two uninfected men.
Those most likely to hold misconceptions about HIV transmission and to harbor HIV/AIDS stigma are people with high levels of religiosity, conservative political ideology and less educated people.[११८][१२०][१२१]
- For more details on this topic, see Stigma and HIV-AIDS, A review of the literature[१२२]
Origin of HIV
[सम्पादन]AIDS was first reported June 5, 1981, when the U.S. Centers for Disease Control and Prevention recorded a cluster of Pneumocystis carinii pneumonia (now classified as Pneumocystis jiroveci pneumonia) in five homosexual men in Los Angeles.[१२३] Health authorities soon realized that nearly half of the people identified with the syndrome were not homosexual men.
Three of the earliest known instances of HIV infection are as follows:
- A plasma sample taken in 1959 from an adult male living in what is now the Democratic Republic of the Congo.[१२४]
- HIV found in tissue samples from a 15 year old African-American teenager who died in St. Louis in 1969.[१२५]
- HIV found in tissue samples from a Norwegian sailor who died around 1976.[१२६]
Two species of HIV infect humans: HIV-1 and HIV-2. HIV-1 is more virulent and more easily transmitted. HIV-1 is the source of the majority of HIV infections throughout the world, while HIV-2 is not as easily transmitted and is largely confined to West Africa.[१२७] Both HIV-1 and HIV-2 are of primate origin. The origin of HIV-1 is the Central Common Chimpanzee (Pan troglodytes troglodytes) found in southern Cameroon.[१२८] It is established that HIV-2 originated from the Sooty Mangabey (Cercocebus atys), an Old World monkey of Guinea Bissau, Gabon, and Cameroon.
Most experts believe that HIV probably transferred to humans as a result of direct contact with primates, for instance during hunting or butchery.[१२९] A more controversial theory known as the OPV AIDS hypothesis suggests that the AIDS epidemic was inadvertently started in the late 1950s in the Belgian Congo by Hilary Koprowski's research into a polio vaccine.[१३०][१३१] According to scientific consensus, this scenario is not supported by the available evidence.[१३२][१३३][१३४]
Alternative hypotheses
[सम्पादन]A small minority of scientists and activists question the connection between HIV and AIDS,[१३५] the existence of HIV itself,[१३६] or the validity of current testing and treatment methods. These claims are considered baseless by the vast majority of the scientific community. The medical community argues that so-called "AIDS dissidents" selectively ignore evidence in favor of HIV's role in AIDS and irresponsibly pose a threat to public health by discouraging HIV testing and proven treatments.[१३७][१३८]
AIDS dissidents assert that the current mainstream approach to AIDS, based on HIV causation, has resulted in inaccurate diagnoses, psychological terror, toxic treatments, and a squandering of public funds.[१३९] Dissident views have been examined and widely rejected,[१४०][१४१] and are considered pseudoscience by the mainstream scientific community.
HIV and AIDS misconceptions
[सम्पादन]A number of misconceptions have arisen surrounding HIV/AIDS. Three of the most common are that AIDS can spread through casual contact, sexual intercourse with a virgin will cure AIDS, and HIV can infect only homosexual men and drug users.
Notes and references
[सम्पादन]- ↑ Marx, J. L. (1982). "New disease baffles medical community". Science 217 (4560): 618–621. PubMed.
- ↑ Divisions of HIV/AIDS Prevention (2003). HIV and Its Transmission. Centers for Disease Control & Prevention. 2006-05-23 कथं।
- ↑ San Francisco AIDS Foundation (2006-04-14). How HIV is spread. 2006-05-23 कथं।
- ↑ Gao, F., Bailes, E., Robertson, D. L., Chen, Y., Rodenburg, C. M., Michael, S. F., Cummins, L. B., Arthur, L. O., Peeters, M., Shaw, G. M., Sharp, P. M. and Hahn, B. H. (1999). "Origin of HIV-1 in the Chimpanzee Pan troglodytes troglodytes". Nature 397 (6718): 436–441. PubMed .
- ↑ ५.० ५.१ ५.२ ५.३ ५.४ ५.५ ५.६ ५.७ ५.८ UNAIDS (2006). “Overview of the global AIDS epidemic”, 2006 Report on the global AIDS epidemic (PDF). Retrieved on 2006-06-08. Cite error: Invalid
<ref>tag; name "UNAIDS2006" defined multiple times with different content - ↑ ६.० ६.१ Palella, F. J. Jr, Delaney, K. M., Moorman, A. C., Loveless, M. O., Fuhrer, J., Satten, G. A., Aschman and D. J., Holmberg, S. D. (1998). "Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators". N. Engl. J. Med 338 (13): 853–860. PubMed.
- ↑ ७.० ७.१ ७.२ ७.३ ७.४ Morgan, D., Mahe, C., Mayanja, B., Okongo, J. M., Lubega, R. and Whitworth, J. A. (2002). "HIV-1 infection in rural Africa: is there a difference in median time to AIDS and survival compared with that in industrialized countries?". AIDS 16 (4): 597–632. PubMed.
- ↑ ८.० ८.१ Clerici, M., Balotta, C., Meroni, L., Ferrario, E., Riva, C., Trabattoni, D., Ridolfo, A., Villa, M., Shearer, G.M., Moroni, M. and Galli, M. (1996). "Type 1 cytokine production and low prevalence of viral isolation correlate with long-term non progression in HIV infection". AIDS Res. Hum. Retroviruses. 12 (11): 1053–1061. PubMed.
- ↑ ९.० ९.१ Morgan, D., Mahe, C., Mayanja, B. and Whitworth, J. A. (2002). "Progression to symptomatic disease in people infected with HIV-1 in rural Uganda: prospective cohort study". BMJ 324 (7331): 193–196. PubMed.
- ↑ Gendelman, H. E., Phelps, W., Feigenbaum, L., Ostrove, J. M., Adachi, A., Howley, P. M., Khoury, G., Ginsberg, H. S. and Martin, M. A. (1986). "Transactivation of the human immunodeficiency virus long terminal repeat sequences by DNA viruses". Proc. Natl. Acad. Sci. U. S. A. 83 (24): 9759–9763. PubMed.
- ↑ Bentwich, Z., Kalinkovich., A. and Weisman, Z. (1995). "Immune activation is a dominant factor in the pathogenesis of African AIDS.". Immunol. Today 16 (4): 187–191. PubMed.
- ↑ १२.० १२.१ Tang, J. and Kaslow, R. A. (2003). "The impact of host genetics on HIV infection and disease progression in the era of highly active antiretroviral therapy". AIDS 17 (Suppl 4): S51–S60. PubMed.
- ↑ Quiñones-Mateu, M. E., Mas, A., Lain de Lera, T., Soriano, V., Alcami, J., Lederman, M. M. and Domingo, E. (1998). "LTR and tat variability of HIV-1 isolates from patients with divergent rates of disease progression". Virus Research 57 (1): 11–20. PubMed.
- ↑ १४.० १४.१ Campbell, G. R., Pasquier, E., Watkins, J., Bourgarel-Rey, V., Peyrot, V., Esquieu, D., Barbier, P., de Mareuil, J., Braguer, D., Kaleebu, P., Yirrell, D. L. and Loret E. P. (2004). "The glutamine-rich region of the HIV-1 Tat protein is involved in T-cell apoptosis". J. Biol. Chem. 279 (46): 48197–48204. PubMed.
- ↑ Kaleebu P, French N, Mahe C, Yirrell D, Watera C, Lyagoba F, Nakiyingi J, Rutebemberwa A, Morgan D, Weber J, Gilks C, Whitworth J. (2002). "Effect of human immunodeficiency virus (HIV) type 1 envelope subtypes A and D on disease progression in a large cohort of HIV-1-positive persons in Uganda". J. Infect. Dis. 185 (9): 1244–1250. PubMed.
- ↑ World Health Organization (1990). "Interim proposal for a WHO staging system for HIV infection and disease". WHO Wkly Epidem. Rec. 65 (29): 221–228. PubMed.
- ↑ Centers for Disease Control (CDC) (1982). "Persistent, generalized lymphadenopathy among homosexual males.". MMWR Morb Mortal Wkly Rep. 31 (19): 249–251. PubMed.
- ↑ Barré-Sinoussi, F., Chermann, J. C., Rey, F., Nugeyre, M. T., Chamaret, S., Gruest, J., Dauguet, C., Axler-Blin, C., Vezinet-Brun, F., Rouzioux, C., Rozenbaum, W. and Montagnier, L. (1983). "Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS)". Science 220 (4599): 868-871. PubMed.
- ↑ १९.० १९.१ Centers for Disease Control (CDC) (1982). "Opportunistic infections and Kaposi's sarcoma among Haitians in the United States". MMWR Morb Mortal Wkly Rep. 31 (26): 353–354; 360–361. PubMed.
- ↑ Altman, L.K.. "'New homosexual disorder worries officials'", New York Times, 1982-05-11.
- ↑ Kher, U.. "A Name for the Plague", Time, 1982-07-27.
- ↑ Centers for Disease Control (CDC) (1982). "Update on acquired immune deficiency syndrome (AIDS)--United States.". MMWR Morb Mortal Wkly Rep. 31 (37): 507–508; 513–514. PubMed.
- ↑ CDC (1992). 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS Among Adolescents and Adults. CDC. 2006-02-09 कथं।
- ↑ २४.० २४.१ Kumaranayake, L. and Watts, C. (2001). "Resource allocation and priority setting of HIV/AIDS interventions: addressing the generalized epidemic in sub-Saharan Africa". J. Int. Dev. 13 (4): 451–466. .
- ↑ Holmes, C. B., Losina, E., Walensky, R. P., Yazdanpanah, Y., Freedberg, K. A. (2003). "Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa". Clin. Infect. Dis. 36 (5): 656–662. PubMed.
- ↑ Guss, D. A. (1994). "The acquired immune deficiency syndrome: an overview for the emergency physician, Part 1". J. Emerg. Med. 12 (3): 375–384. PubMed.
- ↑ Guss, D. A. (1994). "The acquired immune deficiency syndrome: an overview for the emergency physician, Part 2". J. Emerg. Med. 12 (4): 491–497. PubMed.
- ↑ Schneider, M. F., Gange, S. J., Williams, C. M., Anastos, K., Greenblatt, R. M., Kingsley, L., Detels, R., and Munoz, A. (2005). "Patterns of the hazard of death after AIDS through the evolution of antiretroviral therapy: 1984–2004". AIDS 19 (17): 2009–2018. PubMed.
- ↑ २९.० २९.१ Lawn, S. D. (2004). "AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection". J. Infect. Dis. 48 (1): 1–12. PubMed.
- ↑ Campbell, G. R., Watkins, J. D., Esquieu, D., Pasquier, E., Loret, E. P. and Spector, S. A. (2005). "The C terminus of HIV-1 Tat modulates the extent of CD178-mediated apoptosis of T cells". J. Biol. Chem. 280 (46): 38376–39382. PubMed.
- ↑ Senkaali, D., Muwonge, R., Morgan, D., Yirrell, D., Whitworth, J. and Kaleebu, P. (2005). "The relationship between HIV type 1 disease progression and V3 serotype in a rural Ugandan cohort". AIDS Res. Hum. Retroviruses. 20 (9): 932–937. PubMed.
- ↑ Feldman, C. (2005). "Pneumonia associated with HIV infection". Curr. Opin. Infect. Dis. 18 (2): 165–170. PubMed.
- ↑ Decker, C. F. and Lazarus, A. (2000). "Tuberculosis and HIV infection. How to safely treat both disorders concurrently". Postgrad Med. 108 (2): 57–60, 65–68. PubMed.
- ↑ Zaidi, S. A. and Cervia, J. S. (2002). "Diagnosis and management of infectious esophagitis associated with human immunodeficiency virus infection". J. Int. Assoc. Physicians AIDS Care (Chic Ill) 1 (2): 53–62. PubMed.
- ↑ Guerrant, R. L., Hughes, J. M., Lima, N. L., Crane, J. (1990). "Diarrhea in developed and developing countries: magnitude, special settings, and etiologies". Rev. Infect. Dis. 12 (Suppl 1): S41–S50. PubMed.
- ↑ Luft, B. J. and Chua, A. (2000). "Central Nervous System Toxoplasmosis in HIV Pathogenesis, Diagnosis, and Therapy". Curr. Infect. Dis. Rep. 2 (4): 358–362. PubMed.
- ↑ Sadler, M. and Nelson, M. R. (1997). "Progressive multifocal leukoencephalopathy in HIV". Int. J. STD AIDS 8 (6): 351–357. PubMed.
- ↑ Gray, F., Adle-Biassette, H., Chrétien, F., Lorin de la Grandmaison, G., Force, G., Keohane, C. (2001). "Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments". Clin. Neuropathol. 20 (4): 146–155. PubMed.
- ↑ Grant, I., Sacktor, H., and McArthur, J. (2005). “HIV neurocognitive disorders”, H. E. Gendelman, I. Grant, I. Everall, S. A. Lipton, and S. Swindells. (ed.) The Neurology of AIDS (PDF), 2nd, London, UK: Oxford University Press, 357–373. ISBN 0-19-852610-5.
- ↑ Satishchandra, P., Nalini, A., Gourie-Devi, M., Khanna, N., Santosh, V., Ravi, V., Desai, A., Chandramuki, A., Jayakumar, P. N., and Shankar, S. K. (2000). "Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989–1996)". Indian J. Med. Res. 11: 14–23. PubMed.
- ↑ Wadia, R. S., Pujari, S. N., Kothari, S., Udhar, M., Kulkarni, S., Bhagat, S., and Nanivadekar, A. (2001). "Neurological manifestations of HIV disease". J. Assoc. Physicians India 49: 343–348. PubMed.
- ↑ Boshoff, C. and Weiss, R. (2002). "AIDS-related malignancies". Nat. Rev. Cancer 2 (5): 373–382. PubMed.
- ↑ Yarchoan, R., Tosatom G. and Littlem R. F. (2005). "Therapy insight: AIDS-related malignancies — the influence of antiviral therapy on pathogenesis and management". Nat. Clin. Pract. Oncol. 2 (8): 406–415. PubMed.
- ↑ Bonnet, F., Lewden, C., May, T., Heripret, L., Jougla, E., Bevilacqua, S., Costagliola, D., Salmon, D., Chene, G. and Morlat, P. (2004). "Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy". Cancer 101 (2): 317–324. PubMed.
- ↑ Skoulidis, F., Morgan, M. S., and MacLeod, K. M. (2004). "Penicillium marneffei: a pathogen on our doorstep?". J. R. Soc. Med. 97 (2): 394–396. PubMed.
- ↑ Smith, D. K., Grohskopf, L. A., Black, R. J., Auerbach, J. D., Veronese, F., Struble, K. A., Cheever, L., Johnson, M., Paxton, L. A., Onorato, I. A. and Greenberg, A. E. (2005). "Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug Use, or Other Nonoccupational Exposure to HIV in the United States". MMWR 54 (RR02): 1–20.
- ↑ Donegan, E., Stuart, M., Niland, J. C., Sacks, H. S., Azen, S. P., Dietrich, S. L., Faucett, C., Fletcher, M. A., Kleinman, S. H., Operskalski, E. A., et al. (1990). "Infection with human immunodeficiency virus type 1 (HIV-1) among recipients of antibody-positive blood donations". Ann. Intern. Med. 113 (10): 733–739. PubMed.
- ↑ ४८.० ४८.१ Coovadia, H. (2004). "Antiretroviral agents—how best to protect infants from HIV and save their mothers from AIDS". N. Engl. J. Med. 351 (3): 289–292. PubMed.
- ↑ Kaplan, E. H. and Heimer, R. (1995). "HIV incidence among New Haven needle exchange participants: updated estimates from syringe tracking and testing data". J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10 (2): 175–176. PubMed.
- ↑ ५०.० ५०.१ ५०.२ ५०.३ European Study Group on Heterosexual Transmission of HIV (1992). "Comparison of female to male and male to female transmission of HIV in 563 stable couples". BMJ. 304 (6830): 809–813. PubMed.
- ↑ ५१.० ५१.१ ५१.२ ५१.३ ५१.४ ५१.५ Varghese, B., Maher, J. E., Peterman, T. A., Branson, B. M. and Steketee, R. W. (2002). "Reducing the risk of sexual HIV transmission: quantifying the per-act risk for HIV on the basis of choice of partner, sex act, and condom use". Sex. Transm. Dis. 29 (1): 38–43. PubMed.
- ↑ Bell, D. M. (1997). "Occupational risk of human immunodeficiency virus infection in healthcare workers: an overview.". Am. J. Med. 102 (5B): 9–15. PubMed.
- ↑ Leynaert, B., Downs, A. M. and de Vincenzi, I. (1998). "Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV". Am. J. Epidemiol. 148 (1): 88–96. PubMed.
- ↑ Rothenberg, R. B., Scarlett, M., del Rio, C., Reznik, D. and O'Daniels, C. (1998). "Oral transmission of HIV". AIDS 12 (16): 2095–2105. PubMed.
- ↑ Mastro TD, de Vincenzi I (1996). "Probabilities of sexual HIV-1 transmission". AIDS 10 (Suppl A): S75–S82. PubMed.
- ↑ World Health Organization (2006). WHO Multi-country Study on Women's Health and Domestic Violence against Women. 2006-12-14 कथं।
- ↑ Koenig, Michael et al (2004). "Coerced first intercourse and reproductive health among adolescent women in Rakai, Uganda". International Family Planning Perspectives 30 (4:156): 156. }}
- ↑ ५८.० ५८.१ Laga, M., Nzila, N., Goeman, J. (1991). "The interrelationship of sexually transmitted diseases and HIV infection: implications for the control of both epidemics in Africa". AIDS 5 (Suppl 1): S55–S63. PubMed.
- ↑ Tovanabutra, S., Robison, V., Wongtrakul, J., Sennum, S., Suriyanon, V., Kingkeow, D., Kawichai, S., Tanan, P., Duerr, A. and Nelson, K. E. (2002). "Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand". J. Acquir. Immune. Defic. Syndr. 29 (3): 275–283. PubMed.
- ↑ Sagar, M., Lavreys, L., Baeten, J. M., Richardson, B. A., Mandaliya, K., Ndinya-Achola, J. O., Kreiss, J. K., and Overbaugh, J. (2004). "Identification of modifiable factors that affect the genetic diversity of the transmitted HIV-1 population". AIDS 18 (4): 615–619. PubMed.
- ↑ Lavreys, L., Baeten, J. M., Martin, H. L. Jr., Overbaugh, J., Mandaliya, K., Ndinya-Achola, J., and Kreiss, J. K. (2004). "Hormonal contraception and risk of HIV-1 acquisition: results of a 10-year prospective study". AIDS 18 (4): 695–697. PubMed.
- ↑ Cayley, W. E. Jr. (2004). "Effectiveness of condoms in reducing heterosexual transmission of HIV". Am. Fam. Physician 70 (7): 1268–1269. PubMed.
- ↑ Catholic Church (1997). “Offenses against chastity”, Catechism of the Catholic Church : Second Edition. Vatican: Amministrazione Del Patrimonio Della Sede Apostolica, 2353. Retrieved on 2006-06-14.
- ↑ Human Rights Watch (2005). “Restrictions on Condoms”, The Less They Know, the Better. New York NY: Human Rights Watch.
- ↑ Human Rights Watch (2002-09-02). Ignorance only: HIV/AIDS, Human rights and federally funded abstinence-only programs in the United States. Texas: A case study. Human Rights Watch. 2006-03-28 कथं।
- ↑ Debra Hauser (2004). Five Years of Abstinence-Only-Until-Marriage Education: Assessing the Impact (PDF). Advocates for Youth. 2006-06-07 कथं।
- ↑ Durex. Module 5/Guidelines for Educators (Microsoft Word). 2006-04-17 कथं।
- ↑ PATH (2006). "The female condom: significant potential for STI and pregnancy prevention". Outlook 22 (2).
- ↑ WHO (August, 2003). Condom Facts and Figures. 2006-01-17 कथं।
- ↑ The Economist (2005). Too much morality, too little sense. 2006-03-28 कथं।
- ↑ NIAID (2006-12-13). Adult Male Circumcision Significantly Reduces Risk of Acquiring HIV: Trials Kenya and Uganda Stopped Early. 2006-12-15 कथं।
- ↑ Various (2005). Repeated Use of Unsterilized Blades in Ritual Circumcision Might Contribute to HIV Spread in S. Africa, Doctors Say. Kaisernetwork.org. 2006-03-28 कथं।
- ↑ ७४.० ७४.१ Fan, H. (2005). Fan, H., Conner, R. F. and Villarreal, L. P. eds AIDS: science and society, 4th, Boston, MA: Jones and Bartlett Publishers. ISBN 0-7637-0086-X.
- ↑ WHO (2003-03-17). WHO, UNAIDS Reaffirm HIV as a Sexually Transmitted Disease. 2006-01-17 कथं।
- ↑ Physicians for Human Rights (2003-03-13). HIV Transmission in the Medical Setting: A White Paper by Physicians for Human Rights. Partners in Health. 2006-03-01 कथं।
- ↑ United Nations General Assembly (2001). Declaration of Commitment on HIV/AIDS Global Crisis — Global Action. 2006-03-01 कथं।
- ↑ WHO (2001). Blood safety....for too few. 2006-01-17 कथं।
- ↑ Sperling, R. S., Shapirom D. E., Coombsm R. W., Todd, J. A., Herman, S. A., McSherry, G. D., O'Sullivan, M. J., Van Dyke, R. B., Jimenez, E., Rouzioux, C., Flynn, P. M. and Sullivan, J. L. (1996). "Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant". N. Engl. J. Med. 335 (22): 1621–1629. PubMed.
- ↑ Berry, S. (2006-06-08). Children, HIV and AIDS. avert.org. 2006-06-15 कथं।
- ↑ Dias, S. F., Matos, M. G. and Goncalves, A. C. (2005). "Preventing HIV transmission in adolescents: an analysis of the Portuguese data from the Health Behaviour School-aged Children study and focus groups". Eur. J. Public Health 15 (3): 300–304. PubMed.
- ↑ ८२.० ८२.१ Department of Health and Human Services (February, 2006). A Pocket Guide to Adult HIV/AIDS Treatment February 2006 edition. 2006-09-01 कथं। Cite error: Invalid
<ref>tag; name "PEPpocketguide" defined multiple times with different content - ↑ Department of Health and Human Services (February, 2006). A Pocket Guide to Adult HIV/AIDS Treatment February 2006 edition. 2006-09-01 कथं।
- ↑ Department of Health and Human Services Working Group on Antiretroviral Therapy and Medical Management of HIV-Infected Children (November 3, 2005). Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection (PDF). 2006-01-17 कथं।
- ↑ Department of Health and Human Services Panel on Clinical Practices for Treatment of HIV Infection (October 6, 2005). Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents (PDF). 2006-01-17 कथं।
- ↑ Martinez-Picado, J., DePasquale, M. P., Kartsonis, N., Hanna, G. J., Wong, J., Finzi, D., Rosenberg, E., Gunthard, H. F., Sutton, L., Savara, A., Petropoulos, C. J., Hellmann, N., Walker, B. D., Richman, D. D., Siliciano, R. and D'Aquila, R. T. (2000). "Antiretroviral resistance during successful therapy of human immunodeficiency virus type 1 infection". Proc. Natl. Acad. Sci. U. S. A. 97 (20): 10948–10953. PubMed.
- ↑ Dybul, M., Fauci, A. S., Bartlett, J. G., Kaplan, J. E., Pau, A. K.; Panel on Clinical Practices for Treatment of HIV. (2002). "Guidelines for using antiretroviral agents among HIV-infected adults and adolescents". Ann. Intern. Med. 137 (5 Pt 2): 381–433. PubMed.
- ↑ Blankson, J. N., Persaud, D., Siliciano, R. F. (2002). "The challenge of viral reservoirs in HIV-1 infection". Annu. Rev. Med. 53: 557–593. PubMed.
- ↑ Palella, F. J., Delaney, K. M., Moorman, A. C., Loveless, M. O., Fuhrer, J., Satten, G. A., Aschman, D. J. and Holmberg, S. D. (1998). "Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection". N. Engl. J. Med. 338 (13): 853–860. PubMed.
- ↑ Wood, E., Hogg, R. S., Yip, B., Harrigan, P. R., O'Shaughnessy, M. V. and Montaner, J. S. (2003). "Is there a baseline CD4 cell count that precludes a survival response to modern antiretroviral therapy?". AIDS 17 (5): 711–720. PubMed.
- ↑ Chene, G., Sterne, J. A., May, M., Costagliola, D., Ledergerber, B., Phillips, A. N., Dabis, F., Lundgren, J., D'Arminio Monforte, A., de Wolf, F., Hogg, R., Reiss, P., Justice, A., Leport, C., Staszewski, S., Gill, J., Fatkenheuer, G., Egger, M. E. and the Antiretroviral Therapy Cohort Collaboration. (2003). "Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy: analysis of prospective studies". Lancet 362 (9385): 679–686. PubMed.
- ↑ King, J. T., Justice, A. C., Roberts, M. S., Chang, C. H., Fusco, J. S. and the CHORUS Program Team. (2003). "Long-Term HIV/AIDS Survival Estimation in the Highly Active Antiretroviral Therapy Era". Medical Decision Making 23 (1): 9–20. PubMed.
- ↑ Tassie, J.M., Grabar, S., Lancar, R., Deloumeaux, J., Bentata, M., Costagliola, D. and the Clinical Epidemiology Group from the French Hospital Database on HIV. (2002). "Time to AIDS from 1992 to 1999 in HIV-1-infected subjects with known date of infection". Journal of acquired immune deficiency syndromes 30 (1): 81–7. PubMed.
- ↑ Becker SL, Dezii CM, Burtcel B, Kawabata H, Hodder S. (2002). "Young HIV-infected adults are at greater risk for medication nonadherence". MedGenMed. 4 (3): 21. PubMed.
- ↑ Nieuwkerk, P., Sprangers, M., Burger, D., Hoetelmans, R. M., Hugen, P. W., Danner, S. A., van Der Ende, M. E., Schneider, M. M., Schrey, G., Meenhorst, P. L., Sprenger, H. G., Kauffmann, R. H., Jambroes, M., Chesney, M. A., de Wolf, F., Lange, J. M. and the ATHENA Project. (2001). "Limited Patient Adherence to Highly Active Antiretroviral Therapy for HIV-1 Infection in an Observational Cohort Study". Arch. Intern. Med. 161 (16): 1962–1968. PubMed.
- ↑ Kleeberger, C., Phair, J., Strathdee, S., Detels, R., Kingsley, L. and Jacobson, L. P. (2001). "Determinants of Heterogeneous Adherence to HIV-Antiretroviral Therapies in the Multicenter AIDS Cohort Study". J. Acquir. Immune Defic. Syndr. 26 (1): 82–92. PubMed.
- ↑ Heath, K. V., Singer, J., O'Shaughnessy, M. V., Montaner, J. S. and Hogg, R. S. (2002). "Intentional Nonadherence Due to Adverse Symptoms Associated With Antiretroviral Therapy". J. Acquir. Immune Defic. Syndr. 31 (2): 211–217. PubMed.
- ↑ Montessori, V., Press, N., Harris, M., Akagi, L., Montaner, J. S. (2004). "Adverse effects of antiretroviral therapy for HIV infection.". CMAJ 170 (2): 229–238. PubMed.
- ↑ Saitoh, A., Hull, A. D., Franklin, P. and Spector, S. A. (2005). "Myelomeningocele in an infant with intrauterine exposure to efavirenz". J. Perinatol. 25 (8): 555–556. PubMed.
- ↑ १००.० १००.१ १००.२ Ferrantelli F, Cafaro A, Ensoli B. (2004). "Nonstructural HIV proteins as targets for prophylactic or therapeutic vaccines". Curr Opin Biotechnol. 15 (6): 543–556. PubMed.
- ↑ Laurence J. (2006). "Hepatitis A and B virus immunization in HIV-infected persons". AIDS Reader 16 (1): 15–17. PubMed.
- ↑ Fawzi W, Msamanga G, Spiegelman D, Hunter DJ (2005). "Studies of vitamins and minerals and HIV transmission and disease progression". J. Nutrition 135 (4): 938–944. PubMed.
- ↑ Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, Greeson JM, Baum MK, Shor-Posner G, Skyler JS, Schneiderman N. (2007). "Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial". Arch Intern Med. 167 (2): 148–155. PubMed.
- ↑ १०४.० १०४.१ Saltmarsh, S. (2005). "Voodoo or valid? Alternative therapies benefit those living with HIV". Positively Aware 3 (16): 46. PubMed.
- ↑ Mills, E., Wu, P. and Ernst, E. (2005). "Complementary therapies for the treatment of HIV: in search of the evidence.". Int. J. STD AIDS. 16 (6): 395–403. PubMed.
- ↑ UNAIDS (2006). “Annex 2: HIV/AIDS estimates and data, 2005”, 2006 Report on the global AIDS epidemic (PDF). Retrieved on 2006-06-08.
- ↑ UNAIDS (2001). Special Session of the General Assembly on HIV/AIDS Round table 3 Socio-economic impact of the epidemic and the strengthening of national capacities to combat HIV/AIDS (PDF). 2006-06-15 कथं।
- ↑ World Bank (2005). Evaluating the World Bank's Assistance for Fighting the HIV/AIDS Epidemic. 2006-01-17 कथं।
- ↑ CDC (1996). "U.S. HIV and AIDS cases reported through December 1996" (PDF). HIV/AIDS Surveillance Report 8 (2): 1–40.
- ↑ ११०.० ११०.१ ११०.२ Greener, R. (2002). “AIDS and macroeconomic impact”, S, Forsyth (ed.) State of The Art: AIDS and Economics (PDF), IAEN, 49–55.
- ↑ Bollinger, L. and Stover, J. (1999). The Economic Impact of AIDS (PDF). iaen. 2006-03-28 कथं।
- ↑ Over, M. (1992). "The macroeconomic impact of AIDS in Sub-Saharan Africa, Population and Human Resources Department". The World Bank.
- ↑ Bonnel, R. (2000). "HIV/AIDS and Economic Growth: A Global Perspective". S. A. J. Economics 68 (5): 820–855.
- ↑ Bell, C., Gersbach, H. and Devarajan, S. (2003). The long-run economic costs of AIDS: theory and an application to South Africa. eldis. 2006-03-28 कथं।
- ↑ Mu Xuequan (2006). Zimbabwe launches world's first AIDS training package. xinhua. 2006-10-03 कथं।
- ↑ UNAIDS (2006). “The impact of AIDS on people and societies”, 2006 Report on the global AIDS epidemic (PDF). Retrieved on 2006-06-14.
- ↑ Ogden, J. and Nyblade, L. (2005). Common at its core: HIV-related stigma across contexts (पी डी एफ). International Center for Research on Women. 2007-02-15 कथं।
- ↑ ११८.० ११८.१ ११८.२ Herek, G. M. and Capitanio, J. P. (1999). AIDS Stigma and sexual prejudice (पी डी एफ). Am. Behav, Scientist. 2006-03-27 कथं।
- ↑ Snyder M, Omoto AM, Crain AL. (1999). "Punished for their good deeds: stigmatization for AIDS volunteers". American Behavioral Scientist 42 (7): 1175–1192.
- ↑ १२०.० १२०.१ Herek GM, Capitanio JP, Widaman KF. (2002). "HIV-related stigma and knowledge in the United States: prevalence and trends, 1991–1999" (PDF). Am. J. Public Health. 92 (3): 371–377.
- ↑ Herek, GM, Widaman, KF, Capitanio, JP (2005). "When sex equals AIDS: Symbolic stigma and heterosexual adults’ inaccurate beliefs about sexual transmission of AIDS" (PDF). Social Problems. 52 (1): 15–37.
- ↑ United States Health Resources and Services Administration. Stigma and HIV-AIDS, A review of the literature. HRSA. 2006-03-24 कथं।
- ↑ CDC (1981). Pneumocystis Pneumonia — Los Angeles. CDC. 2006-01-17 कथं।
- ↑ Zhu, T., Korber, B. T., Nahmias, A. J., Hooper, E., Sharp, P. M. and Ho, D. D. (1998). "An African HIV-1 Sequence from 1959 and Implications for the Origin of the Epidemic". Nature 391 (6667): 594–597. PubMed .
- ↑ Kolata, G.. "Boy's 1969 death suggests AIDS invaded U.S. several times", The New York Times, 1987-10-28. Retrieved on 2006-06-19.
- ↑ Hooper, E. (1997). "Sailors and star-bursts, and the arrival of HIV". BMJ 315 (7123): 1689–1691. PubMed.
- ↑ Reeves, J. D. and Doms, R. W (2002). "Human Immunodeficiency Virus Type 2". J. Gen. Virol. 83 (Pt 6): 1253–1265. PubMed.
- ↑ Keele, B. F., van Heuverswyn, F., Li, Y. Y., Bailes, E., Takehisa, J., Santiago, M. L., Bibollet-Ruche, F., Chen, Y., Wain, L. V., Liegois, F., Loul, S., Mpoudi Ngole, E., Bienvenue, Y., Delaporte, E., Brookfield, J. F. Y., Sharp, P. M., Shaw, G. M., Peeters, M., Hahn, B. H. (2006). "Chimpanzee Reservoirs of Pandemic and Nonpandemic HIV-1". Science Online 2006-05-25. PubMed.
- ↑ Cohen, J. (2000). "Vaccine Theory of AIDS Origins Disputed at Royal Society". Science 289 (5486): 1850–1851. PubMed.
- ↑ Curtis, T. (1992). "The origin of AIDS". Rolling Stone (626): 54–59, 61, 106, 108.
- ↑ Hooper, E. (1999). The River : A Journey to the Source of HIV and AIDS, 1st, Boston, MA: Little Brown & Co, 1–1070. ISBN 0-316-37261-7.
- ↑ Worobey M, Santiago ML, Keele BF, Ndjango JB, Joy JB, Labama BL, Dhed'A BD, Rambaut A, Sharp PM, Shaw GM, Hahn BH (2004). "Origin of AIDS: contaminated polio vaccine theory refuted". Nature 428 (6985): 820. PubMed.
- ↑ Berry N, Jenkins A, Martin J, Davis C, Wood D, Schild G, Bottiger M, Holmes H, Minor P, Almond N (2005). "Mitochondrial DNA and retroviral RNA analyses of archival oral polio vaccine (OPV CHAT) materials: evidence of macaque nuclear sequences confirms substrate identity". Vaccine 23: 1639–1648. PubMed.
- ↑ Centers for Disease Control and Prevention (2004-03-23). Oral Polio Vaccine and HIV / AIDS: Questions and Answers. 2006-11-20 कथं।
- ↑ Duesberg, P. H. (1988). "HIV is not the cause of AIDS". Science 241 (4865): 514, 517. PubMed.
- ↑ Papadopulos-Eleopulos, E., Turner, V. F., Papadimitriou, J., Page, B., Causer, D., Alfonso, H., Mhlongo, S., Miller, T., Maniotis, A. and Fiala, C. (2004). "A critique of the Montagnier evidence for the HIV/AIDS hypothesis". Med Hypotheses 63 (4): 597–601. PubMed.
- ↑ Cohen, J. (1994). "The Duesberg phenomenon" (PDF). Science 266 (5191): 1642–1644. PubMed.
- ↑ Various (2006-08-13). HIV Science and Responsible Journalism. Seminar at the XVI International AIDS Conference. Kaiser Family Foundation. 2006-06-19 कथं।
- ↑ Various. Controversy. virusmyth.com. 2006-06-19 कथं।
- ↑ Cohen, J. (1994). "The Controversy over HIV and AIDS". Science 266 (5191): 1642–1649.
- ↑ Various. Focus on the HIV-AIDS Connection: Resource links. National Institute of Allergy and Infectious Diseases. 2006-09-07 कथं।

| ||||||||||||||||||||||||||||||
External links
[सम्पादन]| Wikinews has related news: UN/WHO making progress in treating HIV/AIDS, but will miss 2005 target |
- HIV/AIDS resources
- 2006 Report on the Global AIDS Epidemic by UNAIDS — Summary by GreenFacts
- MEDSCAPE.com An extensive site dedicated to everything medical related with an impressive HIV/AIDS section.
- AIDS Educator: Nonprofit making effective AIDS education programs and essential resources widely available to anyone in a position to teach others about HIV/AIDS
- POZ.com Comprehensive resource for the HIV/AIDS community, including all 12 years of POZ Magazine archives.
- AEGiS.org AIDS Education Global Information System
- AVERT AIDS Education & Research Trust from the U.K
- HIV Medicine 2006 Free medical textbook updated annually.
- Eldis HIV and AIDS — latest research and other resources on HIV and AIDS in developing countries
- AIDS.ORG: Comprehensive HIV/AIDS Information
- HIV/AIDS glossary from the U. S. Department of Health and Human Services
- AIDSmeds.com: Comprehensive lessons on HIV/AIDS and their treatments
- The Body: The Complete HIV/AIDS Resource
- AIDS facts and news from the National Institute of Allergy and Infectious Diseases
- HIV and AIDS from New Scientist
- The National Center for HIV, STD, and TB Prevention — A Division of the Centers for Disease Control and Prevention
- AIDS Community Research Initiative of America — Community-based research and education for people living with HIV.
- HIV/AIDS associations and programs
- JOIN(RED) — The Global Fund To Fight AIDS, Tuberculosis and Malaria (Program Launched in the US Friday, 13 October on the The Oprah Winfrey Show)
- The Joint United Nations Programme on HIV/AIDS (UNAIDS)
- International AIDS Society — the world's leading independent association of HIV/AIDS professionals
- Evaluating the World Bank's Assistance for Fighting the HIV/AIDS Epidemic
- Health Action: HIV Transmission in the Medical Setting
- The Global Fund to fight AIDS, Tuberculosis and Malaria (Wikipedia Article)
- IASSTD & AIDS — Indian Association for the Study of Sexually Transmitted Diseases & AIDS
- National AIDS Control Programme (NACP), Tanzania
- Support groups and activism
- Elizabeth Glaser Pediatric AIDS Foundation
- World AIDS Day World AIDS Day 1 December
- World Community Grid — A community supported by Sun Microsystems and IBM. Website has downloadable program which, among other things, uses your PC's spare processing power to analyze how effective a HIV treatment will be.
- Student global AIDS campaign
- Elton John AIDS Foundation
- AIDS Care Education and Training (Wikipedia Article)
- Mercury Phoenix Trust
- TeenAIDS-PeerCorps — focuses on spreading HIV/AIDS education to teenagers around the world
- San Francisco AIDS Foundation
- Documentaries & Reports
- FRONTLINE: The Age of AIDS Twenty-five years after the first cases of AIDS were diagnosed, this program chronicles the scientific, political and social history of the epidemic.
- Documentation on the oral polio vaccine (OPV) theory of AIDS origin AIDSOrigin.com
- Documentary about AIDS in Africa: Living with AIDS
- Origin of Aids Video Watch Free online : Origin of Aids Video
- Recorded interview with Prof. Robert Gallo about the progress in HIV vaccine research
- The Economist : 25 Years — Taking on the epidemic ( Subscription required )
- CBC Digital Archives — The Early Years of the AIDS Crisis
- Documentary "THE GIFT" The Phenomenon of deliberate HIV infection
- "The Graying of AIDS" Older Americans Living with HIV Face Unique Challenges TIME magazine, August 14 2006
